Our Research
Development of methane conversion system
1. Dry reforming of methane using powder-type catalysts
Methane reforming is an important technology which reforms methane that is an abundant resource into
more valuable hydrogen and carbon monoxide. The product, a mixed gas of hydrogen and carbon
monoxide, is called syngas, and is an important material that is used as a raw material for various
industrial products. Although there are multiple gases used for methane reforming, our laboratory is
working on the most difficult reaction using carbon dioxide. In addition, this is a promising
reaction in terms of recycling carbon dioxide which is one of the greenhouse gases causing global
warming. Contrary to the previous research reactions caused by heat, we aim to use renewable energy
like solar light to drive dry reforming of methane.
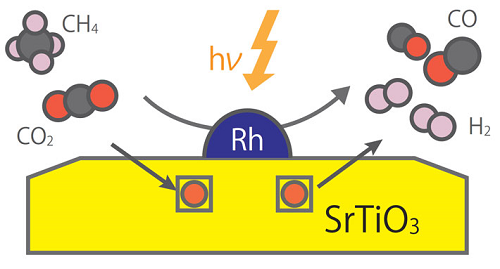
Schematic diagram of dry reforming reaction mechanism by composite photocatalyst Rh/STO
2. Mechanistic investigation utilizing gas-phase photoelectrochemical system
Photocatalysis research was initiated by Honda and Fujishima in the field of electrochemistry, since
then, the concept has been extended to the particulate catalysis systems referred to as
microphotoelectrochemical cells, in which the oxidation and reduction sites are located on a
particle. It is difficult to elucidate the mechanism of the powdered catalysts due to the mixture of
mediating ions, photogenerated electrons, holes, and reaction sites. Therefore, we developed the
gas-phase photoelectrochemical (GPEC) system to separate the oxidation and reduction sites by using
oxygen ions conductors to design a high-performance photocatalyst. Currently, we are studying oxygen
ion mediated dry reforming and steam reforming of methane, and in the future, we plan to expand our
research to other ion mediated reactions.
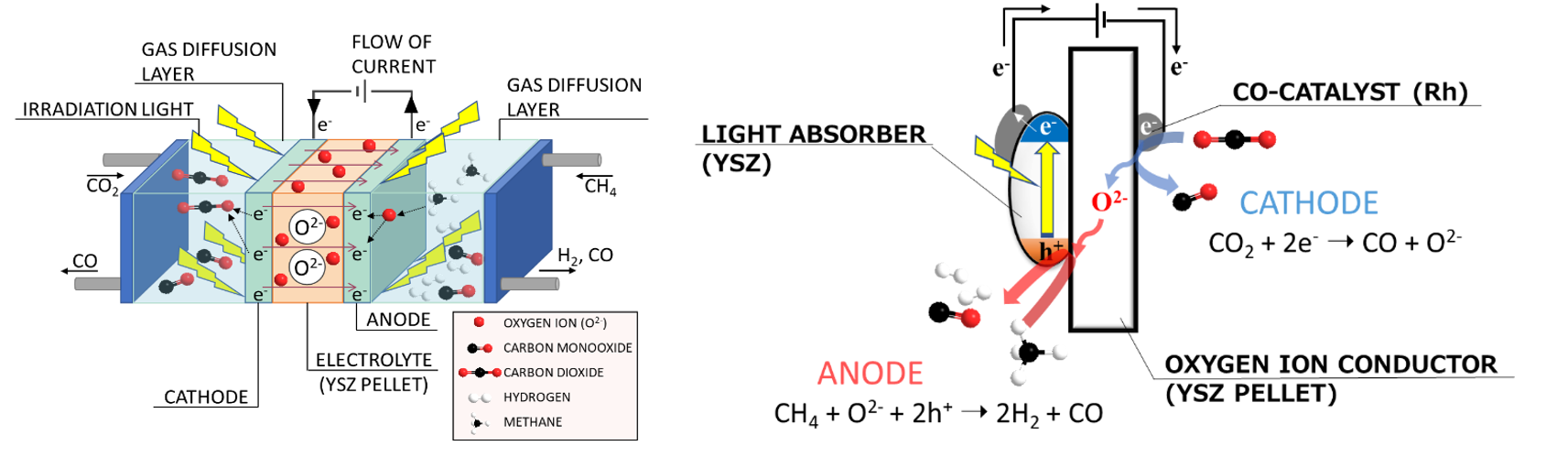
(left)Gas-phase photoelectrochemical (GPEC) system.
(right)Reaction model diagram of methane dry reforming reaction in photoelectrochemical system.
(right)Reaction model diagram of methane dry reforming reaction in photoelectrochemical system.
Development of photo-functional materials
1. HB sheets
Two-dimensional (2D) materials have attracted a lot of attention due to its unique properties. We
are studying boron-based nanosheets which can be synthesized by exfoliating of magnesium diboride
(MgB2) under the collaboration with Prof. Kondo’s group in Tsukuba University. In particular,
hydrogen boride nanosheets (HB sheets), a new 2D material reported recently, is expected to have
various applications because of its unique reducing ability and photoinduced hydrogen release
property.
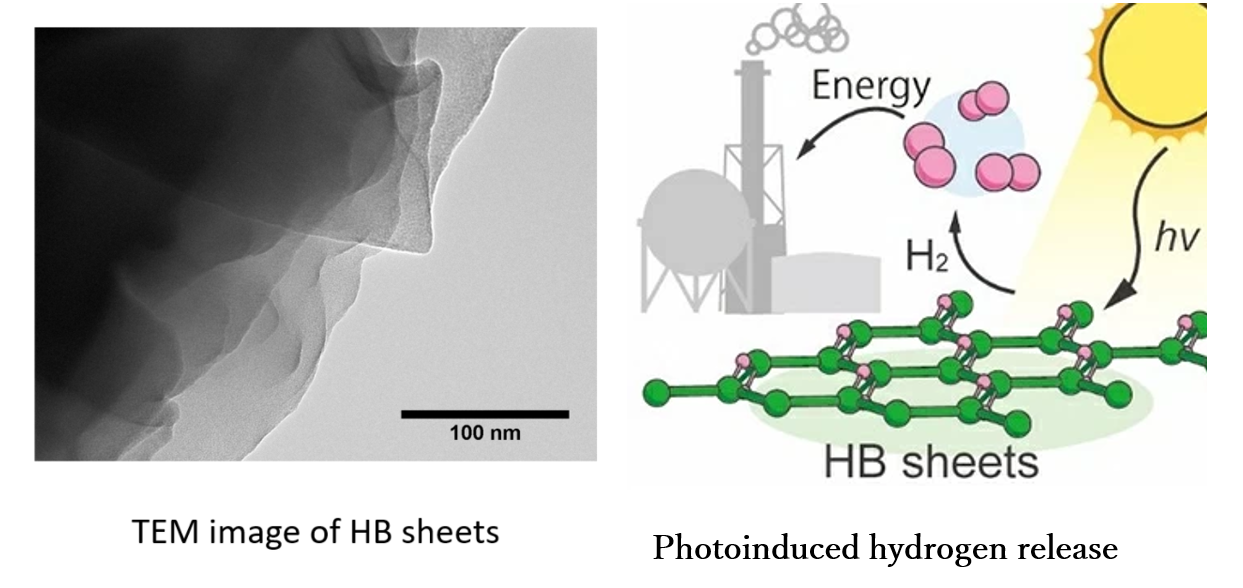
2. Photocatalytic chemical conversion
Photocatalytic water splitting is one of the most attractive ways to harvest the unlimited solar
energy. However, most photocatalysts with desirable band structures are UV light active, which is a
pity because the most intense part of the sunlight is visible light. We are developing the visible
light active photocatalyst with the goal of enhancing the overall photoconversion efficiency. We
currently study the nanoclusters grafted photocatalyst using the concept of interfacial charge
transfer, and mixed valence metal oxide for CO2 reduction. By combining bulk and surface
semiconductor modification, photoelectrochemistry and in-situ measurements, we provide new useful
insights to push forward state-of-the-art of the research, and make great impacts to industry.
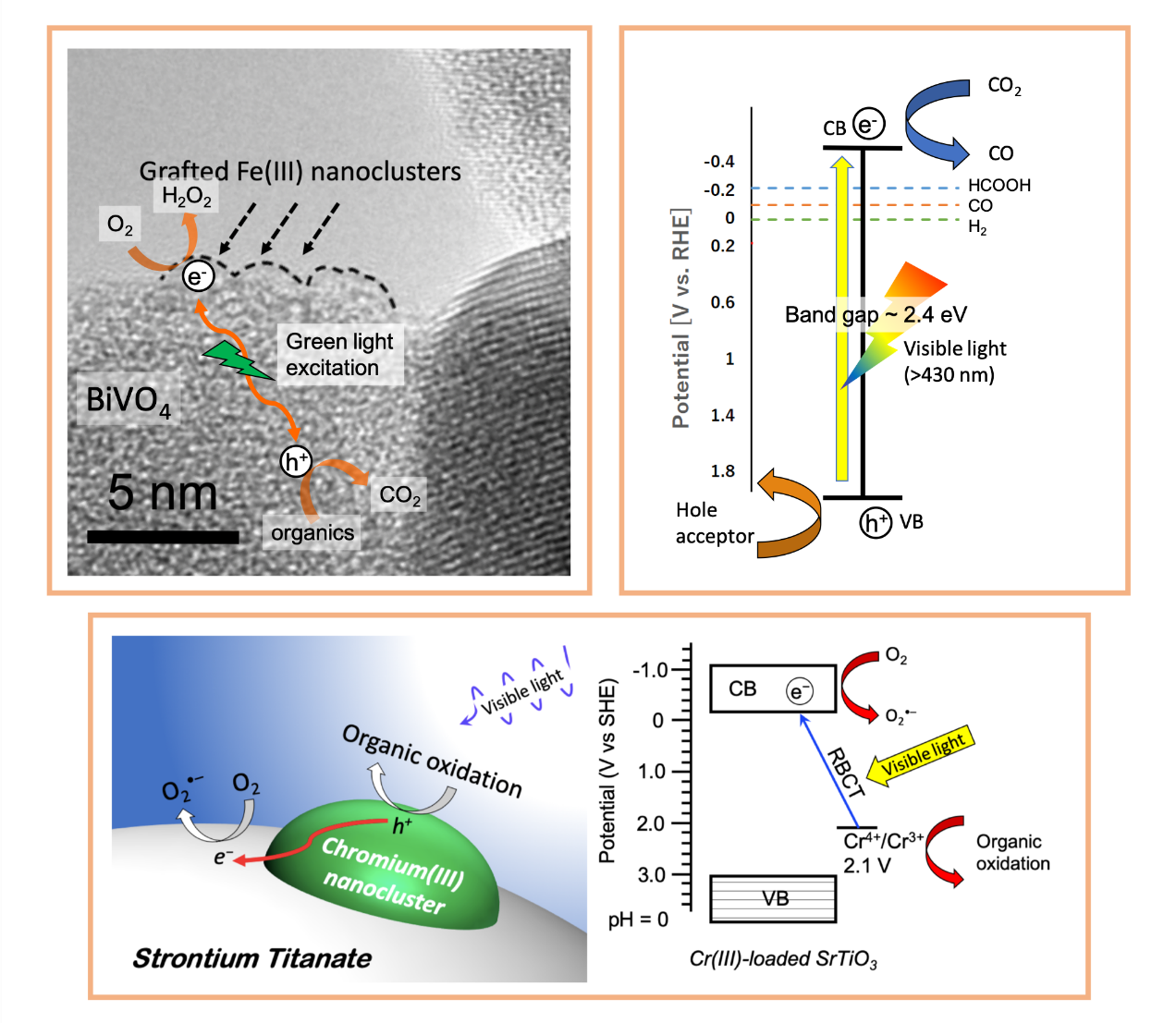
Electrochemical conversion
1. Electrochemical CO2 reduction
Electrochemistry is a promising way for an advance application to overcome carbon waste emission. We
are developing electrode materials and catalysts for electrochemical CO2 reduction, which converts
carbon dioxide into useful products using electricity generated from renewable energy sources and
other sources. We are focusing on metal alloys and metal sulfides as the main electrocatalyst. We
are designing the material by conducting surface modification, tuning morphology and crystal
structure to achieve a better activity. In addition, our group also approaches on computational
study such as machine learning and first principal calculation for a better material design and
prediction of catalytic activity.
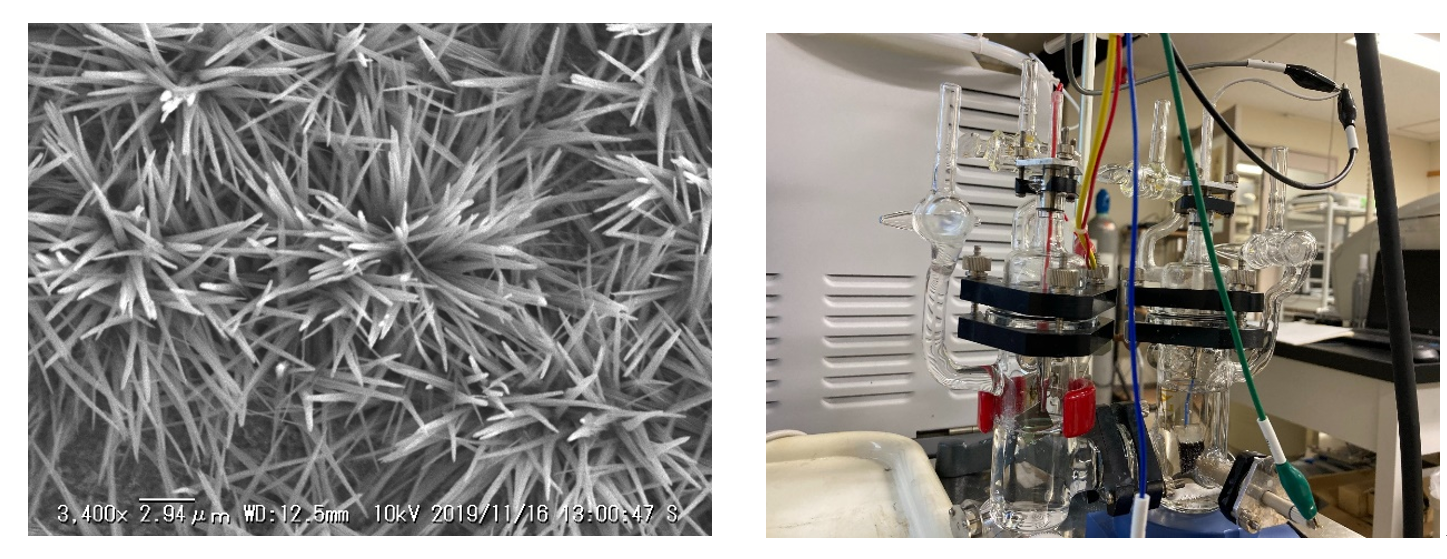
2. Electrochemical Transformation Reactions under Hydrothermal Conditions
Research on traditional electrochemical reactions, especially electrochemical CO2 reduction reactions, has largely been limited to ambient temperature and pressure conditions. Our laboratory focuses on "hydrothermal electrochemistry", conducting reactions at high temperatures and pressures, aiming to improve the efficiency of substance conversion and explore new substances. This apparatus, developed by our laboratory, enables experiments under high temperature and pressure conditions that were previously impossible with conventional electrochemical reactors.
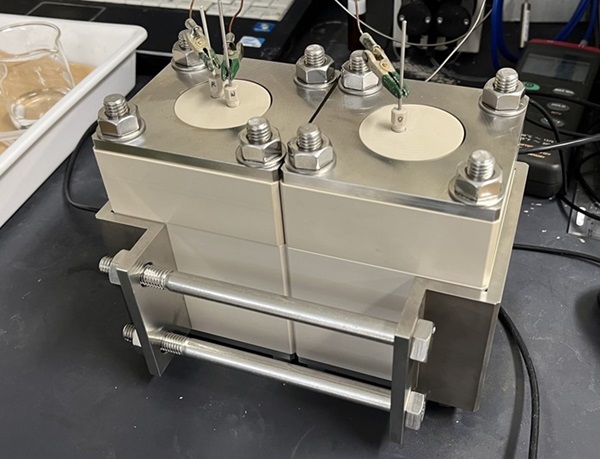
The appearance of HER